Which Best Describes the Current Model of an Atom
Hich best describes how the current scientific model of the atom was developed. The newest understanding of atomic makeup in the Electron Cloud Model better.

Niels Bohr Atomic Theory And Its Limitations Atomic Theory Niels Bohr Chemistry Lessons
The current model of.
. The model was the result of hundreds of years of experiments. The current model of an atom is best described by the Solar System. It is so trusted that no new information could possibly cause.
QUESTION 2 Which of the following statements best describes the current model of the atom. This current atomic model evolved from the earlier Rutherford-Bohr model which compared electrons orbiting an atomic nucleus to planets orbiting the sun. Ernest rutherford performed a famous.
B-it has remained the same for several centuries. Which best describes the current model of the atom. A solid sphere with electrons and protons embedded.
It describes exactly how the atom behaves in every circumstance. It is not an exact representation of the atom but is close enough to be very useful. D- it may be revised with increasing scientific knowledge.
It is not an exact representation of the atom but is close enough to be very useful. A central nucleus containing protons and neutrons with electrons orbiting in levels of high probability. A-it is similar to the model of the solar system with orbiting planets.
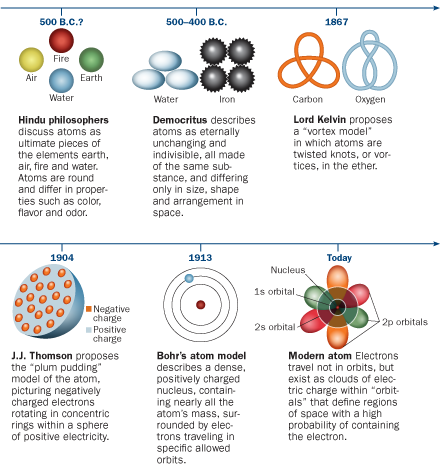
Oh okay In the atomic orbital model the atom consists of a nucleus surrounded by orbiting electrons. Classically the orbits can be likened to the planets orbiting the sun. Nuclear reactions can alter atoms.
Which best describes the current atomic model. The planets represent electrons while. A-it is similar to the model of the solar system with orbiting planets.
A central nucleus containing protons and neutrons with electrons orbiting in levels of high probability. This model is often depicted in artwork showing a central atomic nucleus and oval lines representing the orbits of the electrons. Therefore A C and D being correct statements.
But we know that electrons dont really behave like planets orbiting a central star. A solid sphere with electrons and protons embedded. 5 points The cathode-ray tube experiments showed Thompson that there must be some negative charge in an atom and he figured that it is distributed evenly throughout the atom hence the plum pudding model.
Model of the Atom. Jan 9 2017. Experts are tested by Chegg as specialists in their subject area.
Which best describes the current model of an atom. Which best describes the current model of an atom. Most of the discoveries.
The current model of atomic theory is called the Quantum Mechanical Model otherwise known as the Electron Cloud Model. Correct answer to the question Which best describes the current atomic model. C- it is similar to the model of plum pudding with suspended plums.
Which best describes the current model of an atom. A C and D are the statements that describes Rutherfords model. We can only describe such particles by saying where they will.
Correct answer to the question Which best describes the current model of the atom. Who are the experts. Thomsons cathode-ray tube experiments led to his Plum Pudding model of the atom.
An atom is mostly empty space with a small dense positively charged center. And having a positive nucleus. It is not an exact representation of the atom but is close enough to be very useful.
It is not an exact representation of the atom so it is not very useful. The protons and neutrons are found in the nucleus the large dense center of the atom. It is not an exact representation of the atom so it is not very useful.
Which best describes the current model of the atom. Generally speaking the Bohr model encapsulates the modern understanding of the atom. A solid sphere unique for everything that exists.
Which best describes the current model of an atom. Recent experiments invalidated most of the work of the last 200 years. Which best describes how the current scientific model of the atom was developed 1 point the model was the result of hundreds of years of experiments recent experiments invalidated most of the work of the last 200 years most of the discoveries from the early 1900s were shown to be incorrect new experiments were.
A central nucleus with proton neutrons and electrons orbiting in levels of high probability. Ans4 The current model of the atom is called the quantum model of the atom. The three parts of the atom are protons positively charged neutrons neutral charge and electrons negatively charged.
Protons and neutrons form the atomic nucleus. Q5 Who is the father of atomic theory. An atom is a building block of matter that cannot be broken apart using any chemical means.
B-it has remained the same for several centuries. We review their content and use your feedback to keep the quality high. Rutherfords model shows that an atom is mostly empty space with electrons negative particles orbiting a fixed predictable paths.
A solid sphere unique for everything that exists. Ans5 John Dalton is the father of atomic theory. The electrons are located outside of the the nucleus but the most we can know about their location is the volume of space in which they can be found 90 of the time.
These electrons exist in atomic orbitals which are a set of quantum states of the negatively charged electrons trapped in the electrical field generated by the positively charged nucleus. Which best describes the current atomic model.

Bohr Atomic Model Postulates Distribution Of Electrons Videos Examples

Comments
Post a Comment